Powering Discoveries
Overcoming A Battery's Fatal Flaw
Researchers use supercomputers to develop next-generation lithium-metal batteries for electric vehicles
As renewable energy grows as a power source around the world, one key component has proven elusive: large-scale, stable, efficient, and affordable batteries.
Lithium-ion batteries have been successful for consumer electronics, but electric vehicles, wind turbines, and smart grids require batteries with far greater energy capacity. A leading contender is the lithium-metal battery, which differs from lithium-ion technology in that it contains lithium-metal electrodes.
"Lithium-metal batteries are basically the dream batteries since they provide an extremely high energy density," said Reza Shahbazian-Yassar, associate professor of mechanical and industrial engineering at the University of Illinois at Chicago.
However, engineers have struggled to build commercially viable lithium-metal batteries because of a fatal flaw: dendrites, sharp "needles" of lithium atoms that can cause batteries to heat up, short-circuit, and catch fire.
Recently, a team of researchers, including Shahbazian-Yassar and Perla Balbuena at Texas A&M University, have been inching closer to finding a solution. They used the Stampede1 and Lonestar5 supercomputers to understand the core chemistry and physics at work in dendrite formation and to engineer new materials that can prevent dendrite growth.
Writing in Advanced Functional Materials in February 2018, the researchers presented a new material that may solve the long-standing dendrite problem.
"The idea was to develop a coating material that can protect the lithium metal and make the ion deposition much smoother," said Balbuena, professor of Chemical Engineering at Texas A&M and co-author on the paper.
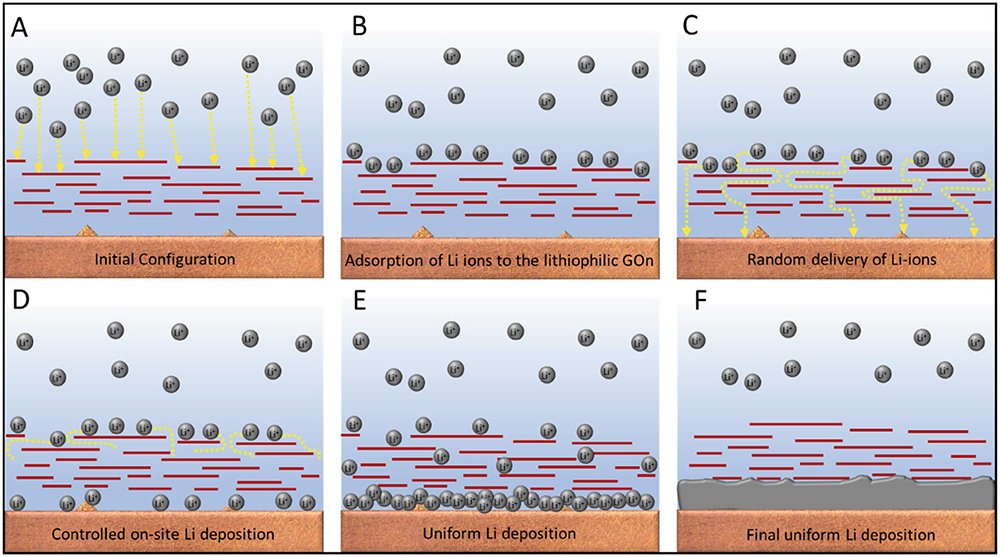
The material the researchers developed is a graphene oxide nanosheet that can be sprayed onto glass and inserted into a battery. The material allows lithium ions to pass through it, but slows down and controls how the ions combine with electrons from the surface to become neutral atoms.
They used computer models and simulations in tandem with physical experiments and microscopic imaging to reveal how and why the material works. The lithium ions, they showed, form a thin film on the nanosheet and then sift through gaps in the layers of the material before settling below the bottom layer of the graphene oxide. The material acts like the pegs in a pachinko game, slowing and directing the metal balls as they fall.
The graphene oxide-doped batteries are stable for up to 160 cycles, whereas an unmodified battery rapidly loses its efficiency after 120 cycles. The oxide can be applied simply and affordably with a spray coating gun.
"The simulations gave our collaborators ideas about the mechanism of ion transfer through the coating," Balbuena said. Some future directions involve different thicknesses or chemical compositions based on the phenomenon that they observed.
The work is supported by the Department of Energy and is aimed at creating smaller, safer, lighter, and less expensive battery packs to make electric vehicles more viable.
"The research is a combination of chemistry, physics, and engineering," said Balbuena, "all enabled by computing, this theoretical microscope that can visualize phenomena through theory."