Powering Discoveries
Jorge Salazar
Related Articles
Plastic Trash to Treasure
Supercomputer simulations help scientists understand enzymes that turn waste into valuable plastic components

One person’s trash can be another’s treasure, as the saying goes. Could this be true in the future for a kind of plastic called polyethylene terephthalate (PET), commonly used to make single-use drink bottles, clothing, food packaging, and carpets?
Thanks in part to supercomputer simulations at TACC, scientists are hopeful about recently-discovered enzymes that break down PET plastic into commercially valuable products. What’s more, the process could feasibly scale up enough to address the global problem of PET plastic pollution.
One of the benefits of PET plastic is resistance to natural degradation. However, this benefit presents a problem after the plastic is used and thrown away. For example, the U.S. Environmental Protection Agency estimates that only about 29 percent of PET bottles are recycled annually.
In 2016, Japanese scientists discovered a bacterium, Ideonella sakaiensis 201-F6, that slowly feeds on PET plastic as its source of carbon and energy. Scientists worldwide pounced on the microbe, studying it in hopes of using that knowledge to engineer organisms that could do it faster.
"We're ideally going from a place where plastics are hard to recycle to a place where we use nature and millions of years of evolution to direct things in a way that makes plastic easy to recycle," said Lee Woodcock, an Associate Professor of Chemistry at the University of South Florida.
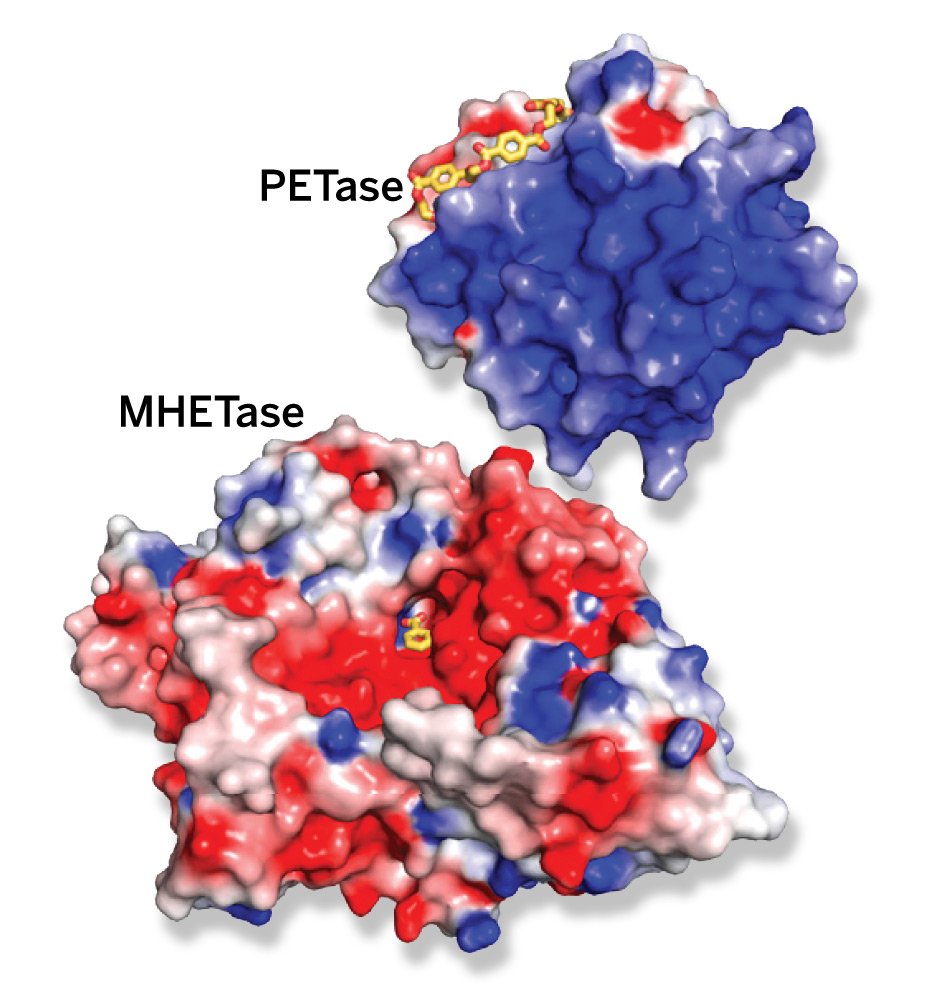
Woodcock co-authored a study published in 2018 in the Proceedings of the National Academy of Sciences (PNAS) that solved the crystal structure of the bacterial enzyme called PETase that degrades PET plastic — a crucial step in developing a biological solution to the pollution problem.
The study authors performed molecular dynamics simulations on TACC’s Stampede2 to capture the interactions of PETase with PET. "We used computer simulations to understand how PET binds to the enzyme," said PNAS study co-author Gregg Beckham, a Senior Research Fellow and Group Leader at the US National Renewable Energy Laboratory (NREL).
Woodcock and Beckham built on this knowledge of PETase to discover a partner enzyme, called MHETase. It basically takes the plastic components that PETase breaks down and cleans them up even further. That’s according to a later PNAS study Woodcock and Beckham co-authored in 2020.
The study found that MHETase coordinates with PETase to form a chimera enzyme. It yields a six-fold improvement in PET depolymerization versus PETase. "There's a synergistic behavior between PETase and MHETase," said study co-author Erika Erickson, a postdoctoral researcher at NREL.
The team did quite a bit of biology and biochemistry to look at how the two enzymes work separately and how they work when they're together. They also employed molecular dynamics simulations on Stampede2 to elucidate the MHETase reaction mechanism. And they studied the effects of covalently binding the two partner enzymes together.
“Coupling computer simulations with wet lab experiments, crys-tal structures, and various other techniques is really powerful."
"Our group has been convinced for a very long time that coupling computer simulations with wet lab experiments, crystal structures, and various other techniques is really powerful," said study co-author Brandon Knott, an NREL staff engineer.
The supercomputer simulations allowed the team to add a dynamic complement to the static crystal structures that otherwise would not have been feasible in their investigation of the reaction mechanisms.
While still far from a solution that deals with the scale of the global plastic waste problem, the scientists see their advances as evidence for hope.
NREL scientists Erickson and Beckham published an impact analysis on enzymatic recycling of PET plastic in the Cell journal Joule, July 2021. The researchers modeled a conceptual enzymatic recycling facility that breaks down PET into its two building blocks, terephthalic acid (TPA) and ethylene glycol. The analysis showed that enzyme-recycled PET could be a potential improvement over conventional, fossil-fuel based methods of PET production, reducing total supply-chain energy use by 69%–83% and greenhouse gas emissions by 17%–43% per kilogram of TPA produced.
"Some companies have started to truly make progress in this direction, that there really could be some enzymatic or biological processes for degrading certain types of plastic waste," Erickson said.